Hydrogen is the most abundant element,
both in the universe and on earth. As an element with the simplest atomic structure
and smallest volume, hydrogen can easily dissolve into many solid materials,
changing their properties. In many industrially important metals during processing
or service, hydrogen often has deleterious consequence on mechanical properties
that is commonly referred to as hydrogen embrittlement (HE), thus has been a wide
concern in industry and academia for over a century.
Despite numerous efforts over the
past century, the exact mechanism of hydrogen effects on the ability of the
material to plastically deform remains controversial, thus knowing how hydrogen
interacts with dislocation – the primary carriers of plasticity is essential. Due
to its high diffusivity, hydrogen is often considered a weak inhibitor or even
a promoter of dislocation movements in metals and alloys.
But our latest experimental discovery
subverts the established cognition in the past decades.
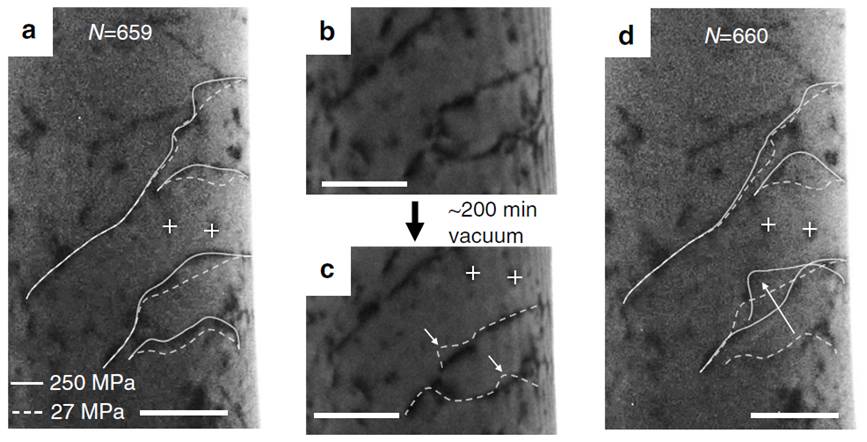
The effect of vacuum aging on dislocation movements in
hydrogen-free sample
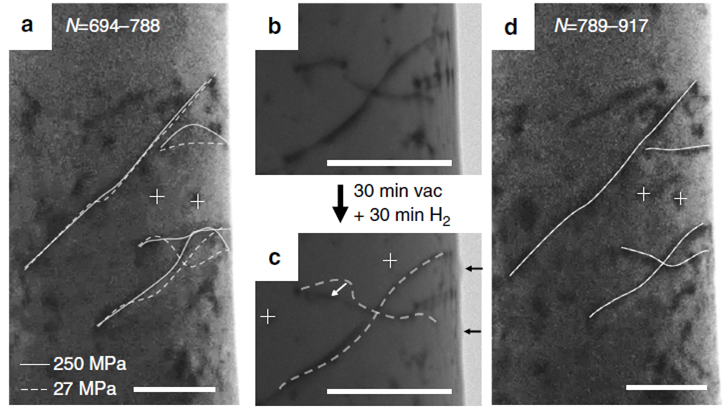
Effect
of hydrogenation on dislocation movements.
By quantitative mechanical tests
in an environmental transmission electron microscope, here Dr. Degang Xie, a young
faculty in CAMP-Nano, demonstrates that after exposing aluminium to hydrogen, mobile
dislocations can lose mobility, with activating stress more than doubled. On
degassing, the locked dislocations can be reactivated under cyclic loading to
move in a stick-slip manner. However, relocking the dislocations thereafter
requires a surprisingly long waiting time of ~103 s, much longer than
that expected from hydrogen interstitial diffusion. Both the observed slow
relocking and strong locking strength can be attributed to superabundant
hydrogenated vacancies, verified by our atomistic calculations. Vacancies
therefore could be a key plastic flow localization agent as well as damage agent
in hydrogen environment.
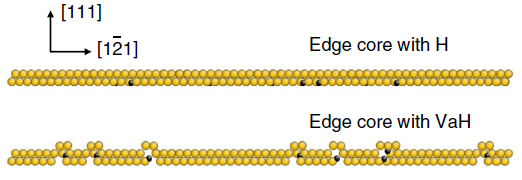
Side view of dislocation core decorated by
hydrogen and hydrogen-vacancy complex, respectively. Atoms with golden and
black colours refer to aluminum and hydrogen, respectively
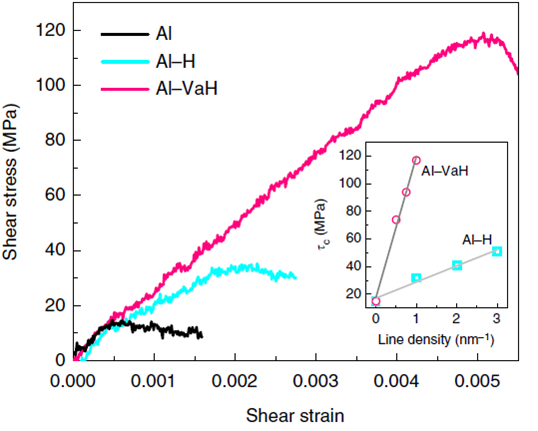
Atomistic
simulation of the pinning effect of hydrogen-vacancy
The project is supervised by Prof.
Zhiwei Shan and Prof. Ju Li. Besides vice Prof. Zhangjie Wang and Dr. Meng Li
from our faculty and post Dr. Suzhi Li, Prof. Peter
Gumbsch, Prof. Jun Sun, Prof. Evan Ma also made a significant contribution to
this work.
This work has been published on
the top research journal, Nature Communications.
The article can be accessed at http://www.nature.com/articles/ncomms13341


