Background
With the increasing attention of researchers on organic electrode materials due to their ability to break free from dependence on transition metal elements, Perylene-3,4,9,10-tetracarboxylic dianhydride (PTCDA) has attracted attention because of its molecular structure consisting of perylene and peripheral oxygen-rich atoms, offering high electronic conductivity. However, like most organic electrode materials, PTCDA faces serious dissolution and shuttling effects in organic electrolytes, which "poison" the active lithium metal anode, ultimately severely affecting the battery's cycling performance and coulombic efficiency. More importantly, this dissolution-precipitation process hinders the study of fundamental scientific issues such as electrode redox reactions and solvent co-intercalation. Therefore, to solve these problems, the ideal solution is to rationally design the electrolyte to achieve quasi-solid-state conversion by inhibiting the intrinsic dissolution of PTCDA, which will lay the foundation for simplifying the redox reaction mechanism, improving battery performance, and exploring fundamental scientific problems.
Introduction
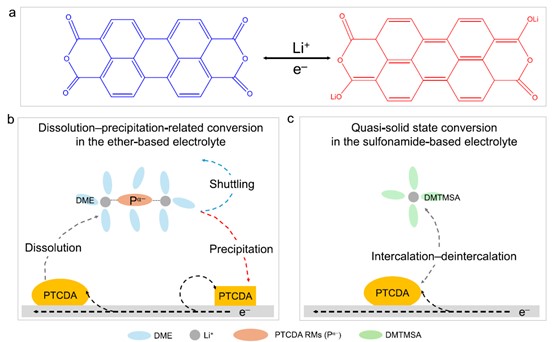
Recently, Professor Xue Weijiang's research group at Center for Advancing Materials Performance from the Nanoscale (CAMP-Nano), State Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University introduced N,N-dimethyltrifluoromethanesulfonamide (DMTMSA) as an electrolyte solvent to address the above issues for PTCDA. By leveraging the weak solvating capability of DMTMSA and its good compatibility with metallic lithium, they designed a 1 M LiFSI/DMTMSA electrolyte, which effectively suppressed the dissolution of PTCDA and successfully achieved their quasi-solid-state conversion. Compared to the gradual decline in energy efficiency (<90%) when using ether-based electrolytes, the sulfonamide-based electrolyte achieved high and stable energy efficiency of up to 95%, owing to the faster kinetics of quasi-solid-state conversion. Additionally, the PTCDA cathode with the sulfonamide-based electrolyte achieved excellent cycling performance (capacity retention ~95.8%) after 300 cycles. Density functional theory calculations and molecular dynamics simulations indicated that co-intercalation of DMTMSA solvent is unlikely to occur in the bulk of PTCDA but may exist at the cathode-electrolyte interface. This work provides a strategy for electrolyte design in long-life lithium-organic batteries and explores fundamental scientific issues such as the kinetics of such cathodes and solvent co-intercalation.

The above research was published on ACS Nano entitled “Quasi-Solid-State Conversion with Fast Redox Kinetics Enabled by a Sulfonamide-Based Electrolyte in Li−Organic Batteries”. Notably, the first author of the paper is Cai Huang, a senior undergraduate student at the School of Materials Science and Engineering, Xi'an Jiaotong University. The Center for Advancing Materials Performance from the Nanoscale (CAMP-Nano), State Key Laboratory for Mechanical Behavior of Materials at Xi'an Jiaotong University are the primary corresponding institutions, with Professor Xue Weijiang as the corresponding author and Professor Tian Chuanjin from Jingdezhen Ceramic University as the co-corresponding author. Other co-authors include Professor Zhou Jian from the School of Materials Science and Engineering, Ph.D. student Cui Xinke, undergraduate student Zhang Yuxin from the School of Energy and Power Engineering, undergraduate student Chen Xinran from the School of Electrical Engineering, and undergraduate student Fan Linghan from the Second Affiliated Hospital of Xi'an Jiaotong University. This work was also supported by the Micro-Nano Center and the Analysis and Testing Center at Xi'an Jiaotong University.
link:https://pubs.acs.org/doi/10.1021/acsnano.4c10343


