Background
With the urgent demand to increase the gravimetric energy density of sodium-ion batteries (SIBs) for practical applications, O3-type layered transition metal oxides have emerged as promising cathode materials due to their high capacity. In particular, raising the cut-off voltage of these cathodes to 4.2 V versus Na/Na⁺ has the potential to achieve energy densities comparable to commercial lithium iron phosphate (LFP)||graphite lithium-ion batteries. However, operating SIBs at such high voltages remains challenging due to severe cathode surface instability, complex phase transformations, and parasitic reactions at both electrodes, especially the risk of sodium metal plating on hard carbon anodes.
A critical barrier lies in designing electrolytes that can support stable operation under such extreme conditions. Existing high-concentration or localized high-concentration ether electrolytes (HCEs/LHCEs) offer some improvements but fall short in suppressing interfacial degradation at high voltages. Thus, the development of a new electrolyte strategy is key to enabling high-performance, high-voltage SIB pouch cells.
Introduction
Recently, Professor Weijiang Xue’s research team from the Center for Advancing Materials Performance from the Nanoscale (CAMP-Nano), State Key Laboratory for Mechanical Behavior of Materials at Xi’an Jiaotong University, in collaboration with Professor Yongsheng Hu’s group from the Institute of Physics, Chinese Academy of Sciences, proposed an innovative electrolyte design: the Intermolecular-Reinforced Electrolyte (IRE). The IRE is formulated by blending two polar solvents—diethylene glycol dimethyl ether (G2) and N,N-dimethyltrifluoromethane-sulfonamide (DMTMSA, abbreviated as DM)—both of which coordinate with Na⁺ ions in the primary solvation sheath.
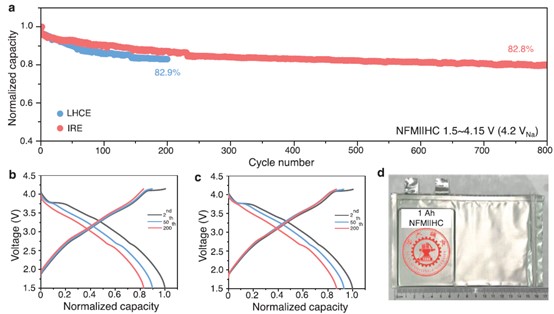
The synergistic interactions between G2 and DM broaden the electrochemical stability window (ESW) and stabilize both the cathode and anode interfaces. In practical 1 Ah-level NaNi₁/₃Fe₁/₃Mn₁/₃O₂||hard carbon (NFM||HC) pouch cells, the IRE enables stable cycling at a high cut-off voltage of 4.2 VNa, achieving an impressive capacity retention of 82.8% after 800 cycles, far outperforming the traditional LHCE-based cells (82.9% after only 200 cycles). This advance establishes a new benchmark for long-life, high-voltage sodium-ion batteries.

The findings were published in Advanced Materials under the title "4.2 V O3‐Layered Cathodes in Sodium‐Ion Pouch Cells Enabled by an Intermolecular‐Reinforced Ether Electrolyte". The first author is Xinke Cui, a Ph.D. candidate at the School of Materials Science and Engineering, Xi’an Jiaotong University. The corresponding authors are Professor Weijiang Xue and Professor Yongsheng Hu. Other co-authors include Shuicen Ding, Yaoshen Niu, Hongkang Wang, and Yaxiang Lu.
This work was strongly supported by the National Key R&D Program of China and benefited from the facilities of the Micro-Nano Center and the Analytical Testing Center at Xi’an Jiaotong University. It provides a forward-looking strategy for electrolyte design, not only enhancing the performance of high-voltage SIBs but also offering fundamental insights into solvation structure, interfacial chemistry, and long-term pouch cell stability.
Paper Link:
https://advanced.onlinelibrary.wiley.com/doi/10.1002/adma.202415611


