Most engineering alloys are crystalline. When crystallization is bypassed by rapid quenching, the resulting alloy—though macroscopically indistinguishable from conventional metals—lacks long-range crystalline order (Fig. 1, left). A wide range of elements and combinations can form amorphous states, including elemental Ta and Au; binary, ternary, and multicomponent systems such as Ni–B, Cu–Zr, Ce–Al, Mg–Cu–Y, Fe–Si–B, Sc–Sb–Te, Pd–Ni–Cu–P, and Zr–Cu–Ni–Al. This breadth opens a parallel design space to crystalline alloys, enabling new materials, functionalities, and processing routes.
For crystalline alloys, the traditional materials-science framework—“lattice plus defects”—links microstructural control (grain size, grain boundaries, dislocations, segregation, twins, stacking faults, precipitates, phase transformations, etc.) to properties. This framework is not applicable to amorphous alloys, whose atomic structure is aperiodic and appears as “maze-like” contrast in high-resolution TEM (Fig. 1). A revised structural perspective is therefore required to establish structure–property relationships for amorphous systems. This foundational problem lacks textbook answers: it presents both a challenge and an opportunity for innovation in materials science.
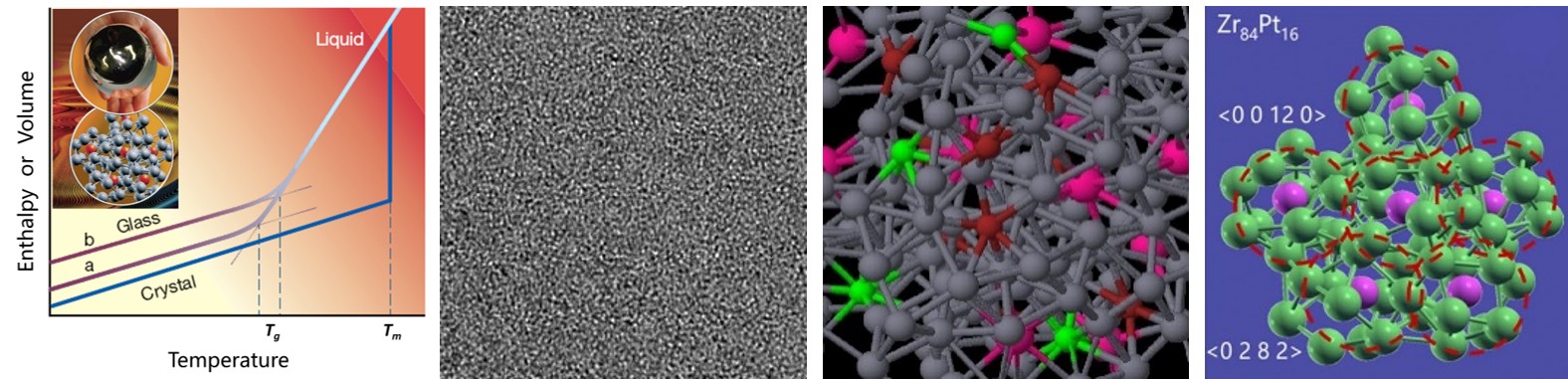
Figure 1. Amorphous alloy produced by liquid quenching and its internal structure. Left: rapid cooling bypasses crystallization to form a glass, which resembles ordinary alloys in appearance yet lacks crystalline order. A representative high-resolution TEM image is shown here, displaying a maze-like pattern. Right: representative local atomic configurations from first-principles molecular dynamics. Short line segments connecting the atoms indicate nearest-neighbor contacts (not directional bonds), forming coordination polyhedra. Different sphere colors denote different elements. For example, Pt is in pink and Zr in green in the right-side panel. Although seemingly random, atoms pack in recurring motifs, as highlighted using dashed contours. These motifs are representative structural building blocks.
Recently, Professor En Ma (CAID, XJTU) published a paper in Advanced Materials entitled “Key structural motifs underlying the unusual deformation flexibility and phase-change propensity in amorphous alloys.” The article synthesizes the group’s systematic analyses of atomic-scale structure in amorphous alloys and, crucially, identifies the key structural motifs that govern distinct classes of properties. By integrating computation, simulation, and advanced characterization, the work delineates families of local configurations—short- and medium-range chemical and topological order—across diverse compositions. Within the manifold of local variants, the study defines and classifies a set of motifs (see an example of recurring coordination polyhedra highlighted in Fig. 1, right), for different alloy systems or composition ratios. This motif-based framework provides a tractable path to solve specific structure–property problems.
Adopting the view that motifs are intrinsically diverse in an amorphous structure, the paper advances the principle of “performance controlled by key structural motifs.” It shows that different deformation behaviors and phase-change propensities in amorphous alloys are each governed by distinct motif types. Building on this core insight, the author proposes motif-targeted strategies to deliberately modify amorphous structures, thereby steering deformation and phase-change mechanisms to achieve responses beyond conventional expectations—and, in some cases, exceeding those of crystalline counterparts.
The paper further clarifies ten foundational questions concerning the heterogeneous structure inside amorphous alloys, offering guidance that addresses common misconceptions and helps readers avoid interpretive pitfalls. The article concludes with ten forward-looking perspectives and recommendations that outline promising research directions.
The author is affiliated with the Center for Alloy Innovation and Design, the State Key Laboratory for Mechanical Behavior of Materials.
Article link: http://doi.org/10.1002/adma.202515726


