Poly(lactic acid) (PLA) is meant to be the prime biodegradable and biosourced substitute for classic polymers such as polypropylene. But crystallization of its desirable alpha-form is too slow for fast commercial processing, even slowing down instead of accelerating with increasing supercooling. So products end up brittle in the inferior alpha’ form with low crystallinity. The crystallization slow-down is now explained by a new phenomenon called “polymorphic self-poisoning”. In alpha-form chains follow a strict up-down order, while in the less stable alpha’ orientation is random. But the polymer molecules don’t “know” that, and on cooling, as alpha’ is nearing stability, the randomly attached chains “think” they are already stable. So they linger at the surface of alpha crystal blocking its growth, before eventually melting away. “Poisoning” is the term used for crystal growth blocked by attached impurities. But here the “impurities” are the native molecules, just in wrong orientation. While small molecules in melt change orientation easily, polymer chains do not (Figure 1). Suggested solution: design symmetric chain plastics. Preliminary work suggests that “polymorphic self-poisoning” also happens in other polymers, particularly at high supercooling encountered in fast commercial processing.
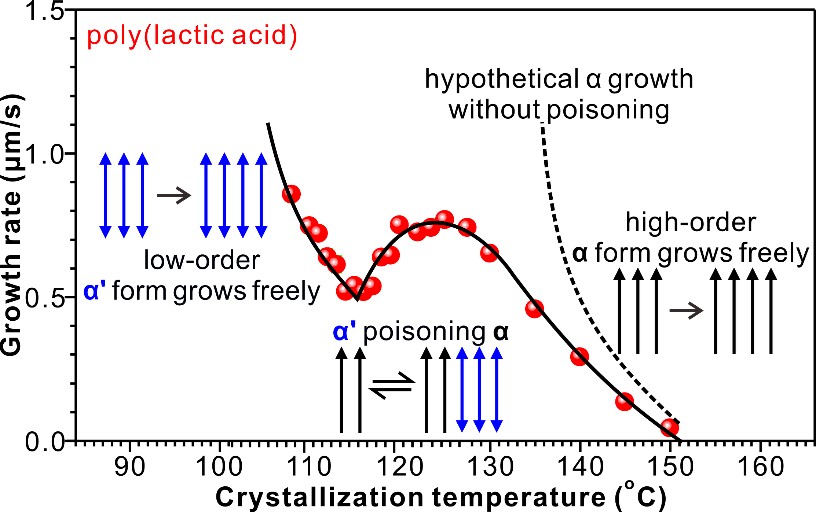
Figure 1. Spherulite radial growth rate vs crystallization temperature, and schematic of polymorphic self-poisoning
Link for the paper: https://journals.aps.org/prl/abstract/10.1103/yf56-tfhd


